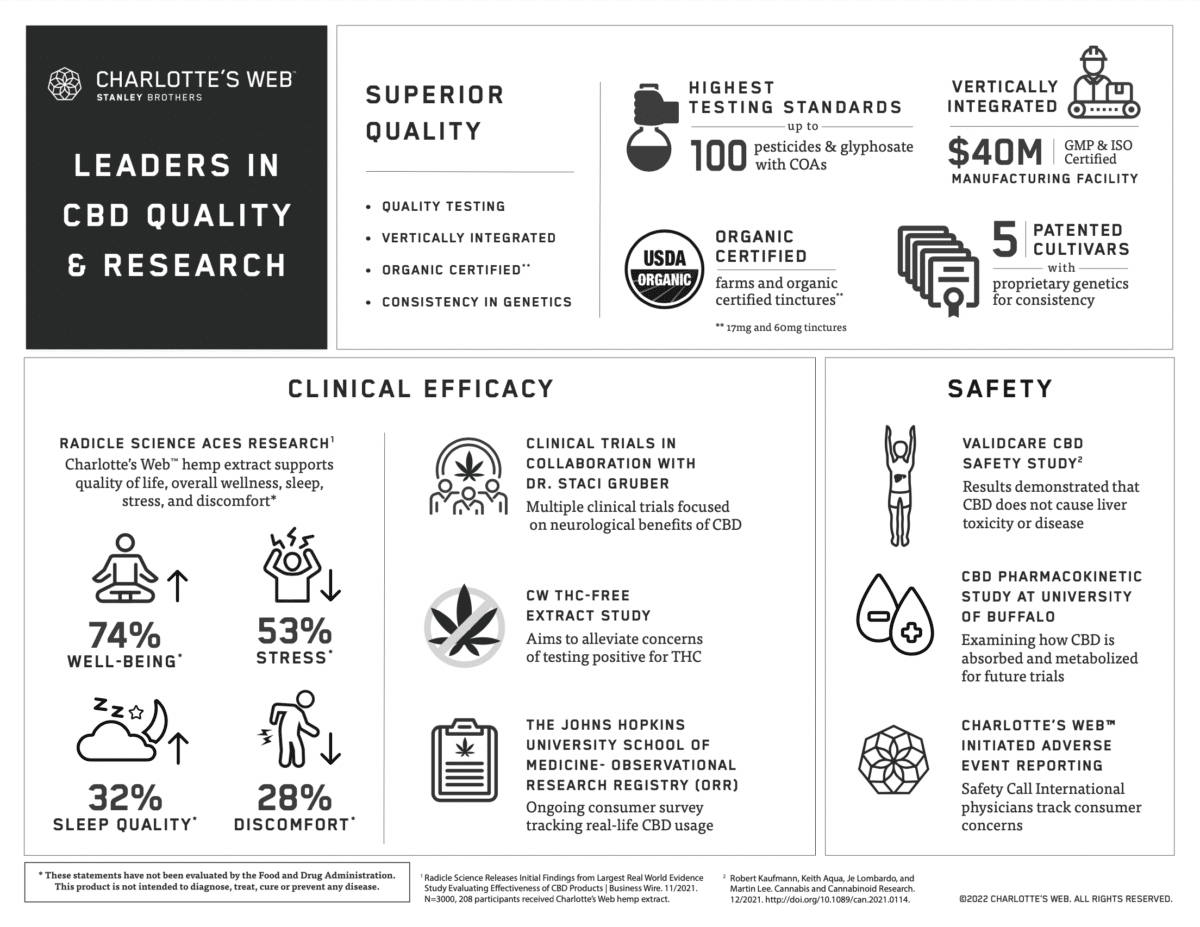
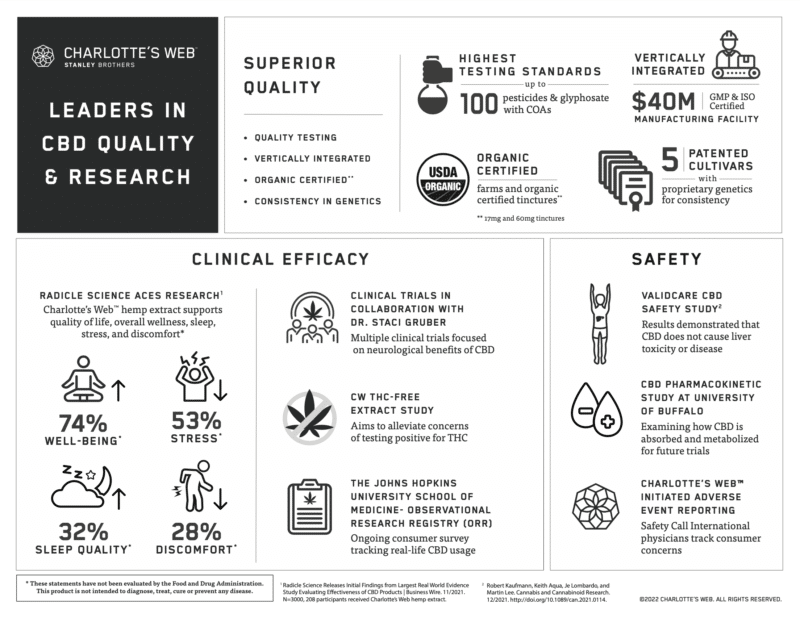
Charlotte’s Web prioritized brand excellence from the very beginning. The same steps the company took to establish itself as an industry leader in quality and integrity – for example, a premium caliber, vertically integrated supply chain; five patents for plants with consistent, proprietary hemp genetics – also mean Charlotte’s Web full spectrum hemp extracts and hemp-derived CBD products have documented purity and batch-to-batch consistency. The company’s commitment to science is demonstrated through its own research and development division (CW Labs), as well as its engagement in studies conducted by independent investigators. Here are highlights of those studies.
Safety
Third-Party Tracking of Consumer Concerns
Charlotte’s Web willingly adheres to the safety reporting standards upheld by the 1994 Dietary Supplement Health Education Act (DSHEA). We engage the services of SafetyCall International, an independent authority with the know-how to track and categorize consumer-reported adverse events and complaints in the health and wellness arenas. A paper summarizing 18 months of data showing an excellent history of safety for Charlotte’s Web products is expected in late 2022.
CBD and Liver Function
Charlotte’s Web participated in the Validcare study examining potential effects of CBD on liver enzymes. Liver testing was performed on 839 participants aged 18-75 who took CBD orally for a minimum of 30 days. The study found no evidence of liver disease, and no increased prevalence of elevated liver function tests when compared to a population with a similar incidence of medical conditions.1 The next phase of this study is ongoing.
CBD Pharmacokinetics
In a pilot study conducted by the University of Buffalo’s Center for Integrated Global Biomedical Sciences, participants took 50 mg/1 mL of Charlotte’s Web Original Formula for six days. CBD levels were measured every hour for eight hours on days 1 and 6. Preliminary data from this study will lay the groundwork for future studies of CBD absorption and metabolism.
Real World Effectiveness
Radicle Science ACES (Advancing CBD Education and Science)
As part of a large, open label, randomized controlled trial, 208 participants took an average of 1.8 servings (one serving = 50 mg CBD) per day of Charlotte’s Web Original Formula for four weeks. Primary outcomes were “clinically meaningful changes,” defined as “distinct and palpable improvements in quality of life” across the following wellness domains: sleep, stress, discomfort, and general well-being. Participants were assessed at baseline and regularly throughout the four-week study period. Findings for the Charlotte’s Web study arm showed: Sleep Quality improved on average 32% from baseline. 60% of participants with sleep issues reported clinically meaningful improvements in sleep quality as compared to 15% of controls. Stress decreased on average 53% from baseline. 64% of participants with stress issues reported clinically meaningful improvements as compared to 22% of controls. Discomfort decreased on average 28% from baseline. 51% of participants with discomfort reported clinically meaningful improvements. General Well-Being improved by 74%. Original Formula worked quickly, with effect onset reported on average within four hours, and notable improvements occurring across all health outcomes on average within the first week.
Partnership with Johns Hopkins University and Realm of Caring Observational Research Registry
Since 2016, this large observational cohort study has collected real-world data on how people use cannabinoid products, including benefits experienced, adverse events, and drug interactions. We apply insights provided by data on the use of Charlotte’s Web products to deepen our understanding of dosing, safety, and effectiveness. One peer-reviewed paper has already been published on CBD and neurological health. More papers will be forthcoming.
THC Free Assurance Study
Charlotte’s Web is in the process of conducting a study to confirm that our THC Free extracts can be used with confidence by people whose employment requires they be subject to routine drug screening.
Clinical Efficacy
Partnership with Harvard Medical School
Charlotte’s Web is collaborating on two clinical trials with Staci A Gruber, PhD, Associate Professor of Psychiatry at Harvard Medical School, and Director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital. In addition, Dr. Gruber is conducting a cohort study with Veterans who use Charlotte’s Web products. More information on these trials will be forthcoming.
Full Infographic: Charlotte’s Web Leaders in CBD Quality & Research
> Click here to download infographic